Need for Substantially Increased Testing Capacity
Commitments on therapeutics must be supported by a corresponding increase in timely testing capacity. Effectiveness of the antivirals requires that they are administered within a limited time period after the start of symptoms. Consequently, effective use will require enhanced access to diagnostic testing and timely reporting of results, ideally pairing rapid testing with access to drugs. In addition, testing is needed for screening in settings that face high-risk outbreaks. Without significantly increased testing capacity, countries will continue to struggle to contain spread and risk becoming super-incubators.
The international COVID-19 diagnostics and testing landscape is complex; there are multiple dimensions to consider in assessments of testing capacity and need, such as type of test (e.g., PCR versus rapid antigen), diagnostic versus screening and surveillance testing, and requirements for test administration. Around the world, even among high-income countries, proposed targets and approaches vary significantly.
The Global COVID-19 Access Tracker confirms that there is alignment from ACT-A, MLTF, and the Global COVID Summit on a target for a daily testing rate of 1 in 1,000 people in all countries (also stated as 100 in 100,000 in the ACT-A strategy). This testing rate is less than one-seventh the current rate in high-income countries, highlighting the scale of global disparities.
Data from FIND, a global alliance for diagnostics and co-lead of the ACT-A diagnostics pillar, indicate that many countries are far from meeting this target. Not surprisingly, big gaps in the data exist – almost half of all sub-Saharan African countries do not have available testing data on the FIND tracker.
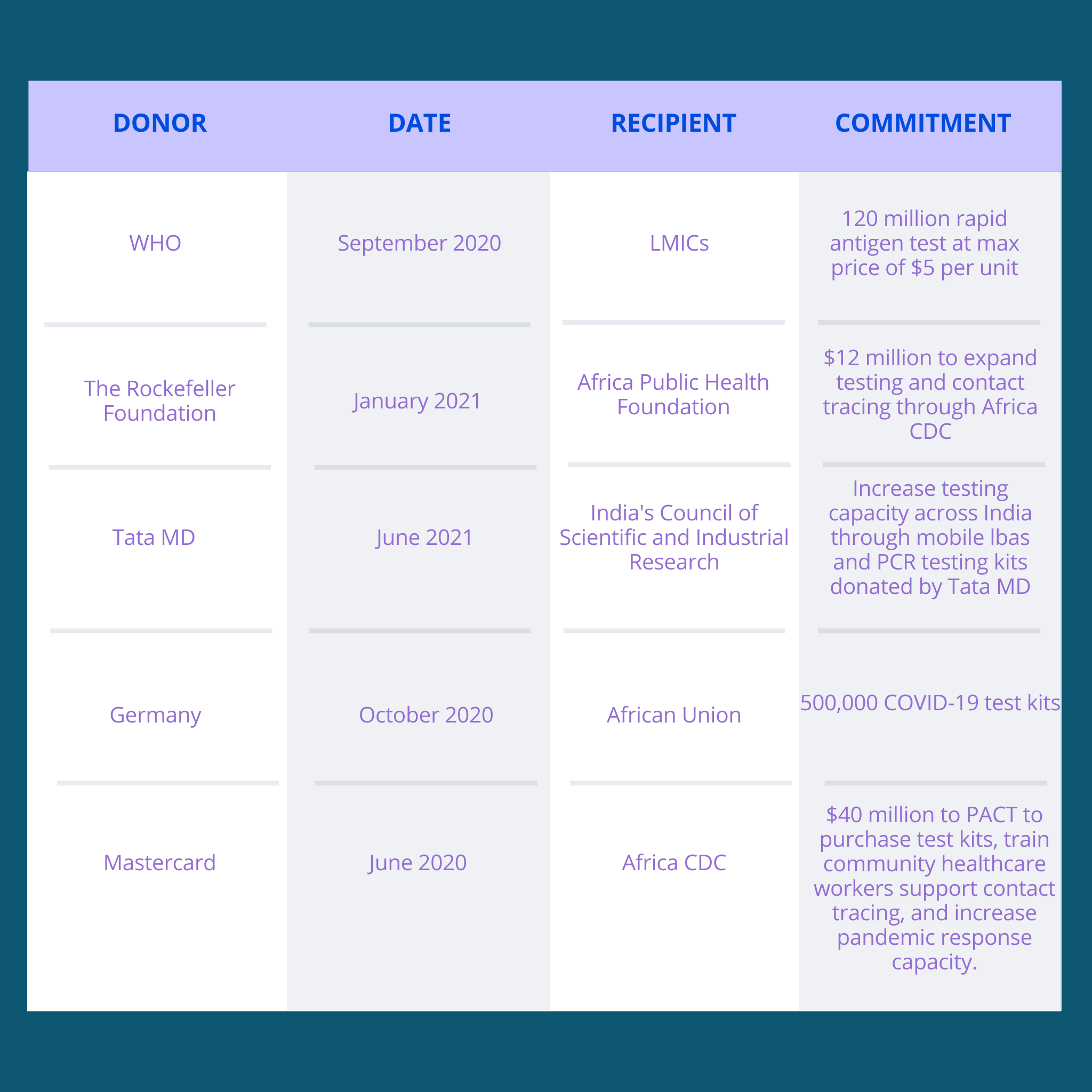
The ACT-A strategy set the following targets: procuring 988 million tests over the next 12 months, supporting the development and local manufacturing of three new point of care tests, and expanding genomic sequencing capacity to reach at least 75 percent of countries. The ACT-A diagnostics pillar estimates it will need $7 billion to execute its strategy, with the majority of the budget allocated for procurement, including for approximately 600 million tests. However, the total funding needed to increase diagnostic capacity is not known.
Consequently, more data should be obtained to assess whether commitments and funding for increased testing capacity will provide reasonable access to timely testing, a topic that COVID GAP will address in the coming weeks. In the meantime, to increase testing capacity, there is need for additional funding for capacity development in laboratories supported by PEPFAR and other global programs, and increased procurement and deployment of rapid point-of-care tests authorized in G7 and EU countries as manufacturing capacity rises.